i3 -1 lewis structure|Lewis Structure for I3 : Tuguegarao Lewis structure is the representation of the electrons of the molecules. There are lone pairs and valence electrons which help . There are 993 Georgia Jumbo Bucks Lotto drawings since February 23, 2015. Note: Lottery Post maintains one of the most accurate and dependable lottery results databases available, but errors can .
PH0 · Triiodide ion (I3
PH1 · Lewis Structure of I3
PH2 · Lewis Structure for I3
PH3 · I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity,
PH4 · I3
Description: Honey Hayes and her stepbrother are on vacation with their family for Memorial Day - but there’s a small problem with accommodations.Forced to spend their vacation in a place with only one bedroom, Honey and her stepbro get a .
i3 -1 lewis structure*******A step-by-step explanation of how to draw the I3 - Lewis Dot Structure (Triiodide Ion). For the I3 - structure use the periodic table to find the total number of valence electrons for the.i3 -1 lewis structure Lewis Structure for I3 Lewis structure is the representation of the electrons of the molecules. There are lone pairs and valence electrons which help .
I3- Lewis Structure - Triiodide Ion. This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and.
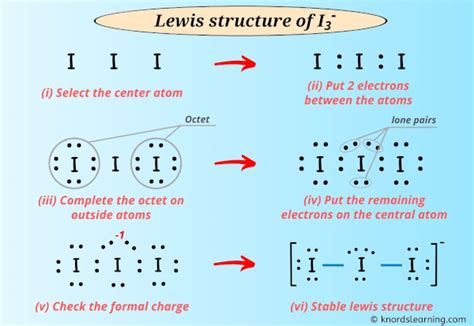
Lewis Structure for I3-. Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact .
What is the Lewis structure of I3-? The Lewis structure of I3- represents the triiodide ion. It consists of three iodine atoms (I) bonded in a linear arrangement (I-I-I) with two lone . Lewis structure of I3- ion (triiodide) contains two single bonds between each Iodine (I) atom. All the three Iodine atoms have three lone pairs on it, and the .
The Lewis structure shows two single I-I bonds and three lone pairs on the central I atom. The end iodine atoms each have three lone pairs. I3⁻ exhibits a linear .Each iodine atom has 3 lone pairs and center iodine atom have -1 charge. We will learn how to draw the lewis structure of I 3- step by step in this tutorial. I 3- lewis structure. .
In the I 3– Lewis structure, there are two single bonds around the iodine atom, with two other iodine atoms attached to it, and on each iodine atom, there are .
I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.
3. -. ) - Lewis Structure. In the lewis structure of triiodide ion (I 3- ), there are two I-I bonds. and one iodine atom is located as the center atom. Each iodine atom has 3 lone pairs and center iodine atom have -1 charge. We will learn how to draw the lewis structure of I 3- step by step in this tutorial. This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and form.
If we use the formula to place or substitute the values, we get. 7+1+2/2. =10/2. =5. As a result, the hybridisation number is 5. Hybridisation is now classified as sp3d. Alternatively, knowing the number of valence electrons and lone pairs and computing their sum can be used to calculate the Hybridization of I3-.

Triiodide [I3]- ion Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, polar vs non-polar. The chemical formula I 3– represents an anion composed of three iodine (I) atoms. This anion is known as the triiodide ion. The triiodide (I 3–) ion is generated by the chemical reaction .Lewis Dot of the Triiodide Ion. I 3-. Back. 70 More Lewis Dot Structures. I does not follow the octet rule. It will hold more than 8 electrons. Iodine having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons. I 3- is dsp 3 hybridized and contains 3 lone pairs and 2 . Hello Guys,We are back with one of the most requested videos on Geometry of Molecules- I3- Lewis structure. It is a chemical formula for the Triiodide ion. T.
Drawing the Lewis Structure for I 3-Viewing Notes: I 3-has a negative charge (and is therefore a negative ion or anion). That means that it has an extra electron that needs to be taken into account. . Let's do the I3- Lewis structure. On the periodic table, Iodine is in group 7 or 17. It has seven valence electrons. But we have three of them . Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
i3 -1 lewis structure I3- Estructura de Lewis, Geometría, Hibridación: 7 pasos (resuelto) El ion triyoduro (I3⁻) consta de una disposición lineal de tres átomos de yodo (I), con el átomo I central unido a dos átomos I terminales. Tiene 7 electrones de valencia por átomo, más un electrón adicional debido a la carga negativa, totalizando 22 electrones. QUIMICA Estructura de Lewis I3 (-1) hibridación carga formal y geometría AULAEXPRESS3. -. ) - Lewis Structure. In the lewis structure of triiodide ion (I 3- ), there are two I-I bonds. and one iodine atom is located as the center atom. Each iodine atom has 3 lone pairs and center iodine atom have -1 charge. We will learn how to draw the lewis structure of I 3- step by step in this tutorial. 1. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. The Lewis electron structure is. 2. There are two electron groups around the central atom. We see from Figure 9.2 that the arrangement that minimizes repulsions places the groups 180° apart. 3.
I3- is formed by the bonding of I2 with I− ion. During the combination of Iodine atoms, the central atom gains a negative charge whose value will be 1. I− ion is the donor and I2 molecule is the acceptor. Electrons are mostly accommodated in the empty d orbitals. I3- Molecular Geometry And Bond Angles. I3- molecular geometry is linear.2. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons. 3. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over.Lewis Structure for I3 Cette charge globale -1 sur la molécule I3 est représentée dans l’image ci-dessous. Dans la structure de points de Lewis ci-dessus de l’ion I3-, vous pouvez également représenter chaque paire d’électrons de liaison (:) comme une liaison simple (|). Ce faisant, vous obtiendrez la structure de Lewis suivante de I3-ion. Ici je 3-Lewis structure hybridation = (7+2+1)/2 = 5 c'est-à-dire, sp 3 d. . Ion triiodure, I3-Structure de Lewis, est un ion polyatomique linéaire avec sp 3 d hybridation et géométrie trigonale bipyramidale et acquisition d'une forme linéaire connue par le modèle VSEPR.
Disaster Arena fue creado por emolingo games. Juega a sus otros juegos en Poki: Escape From School, Sword Masters y Rainbow Obby! ¿Cómo puedo jugar Disaster Arena gratis? Puedes jugar a Disaster Arena gratis en Poki. ¿Puedo jugar Disaster Arena en dispositivos móviles y computadoras de escritorio?
i3 -1 lewis structure|Lewis Structure for I3